Intellectual property is at the center of U.S. biotech leadership. It plays an essential role in the development of lifesaving scientific advances - and the safeguarding of America's national security.
Keeping America Competitive
Strong patents, and an efficient, predictable, and objective patent system, are critical to ensuring that America continues to leads the world in innovation, providing the United States with a global competitive advantage and spurring economic growth and the creation of high-paying jobs here at home.
Creating Breakthroughs
Strong, reliable IP protections allow startups to turn breakthrough science into lifesaving treatments — which is no easy feat. Roughly nine in 10 medicines ultimately fail in clinical trials. Without IP, the financial risks of drug development would be insurmountable.
Putting U.S. Patients First
A strong patent system ensures that U.S. patients continue to have priority access to first-in-class treatments — as well as the best and newest formulations of existing medicines. On average, new drugs are launched in the U.S. a full year before they hit other major markets like Germany and Canada.
The Lifeblood of U.S. Innovation Leadership
Safeguarding National Security
A robust, predictable IP system fuels the drug development cycle — protecting discoveries, incentivizing risk-taking, and driving the next generation of breakthroughs. By safeguarding IP, we safeguard America's global leadership, ensuring we'll continue to out-innovate China. Over the past decade, China has increased its biopharma R&D spending 400-fold.
Spurring Capital Formation
For biotech startups, IP is often the only asset that attracts the investment needed to fund early-stage innovation. Weakening IP protections chokes off this critical funding pipeline — and advantages foreign competitors. "Biotech firms with robust patent portfolios attract investment 4-5 times faster than those without strategic IP protection," according to global industry data.
Strengthening Economic Growth
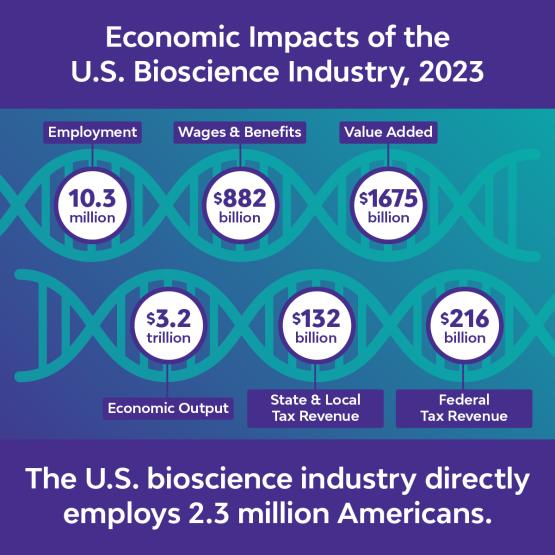
IP protections underpin the biotech sector’s ability to operate and to fuel economic growth, offer high-paying jobs, and revitalize local communities. The U.S. bioscience industry supports over 10 million jobs and $3 trillion in total economic output.
IP and the "Virtuous Circle" of Innovation
Advancing newer and better medicines, therapeutics, and vaccines is a collective effort that involves an entire ecosystem, not just one person or company. This virtuous circle of innovation often begins with a patient or family expressing a need, and is followed by researchers responding with innovative ideas. BIO's role is to protect, advance, and enlarge this innovation ecosystem.
"The role of BIO is going to be to make sure that that entire innovation ecosystem, that virtuous circle, is protected and advanced.”
- BIO President & CEO John F. Crowley

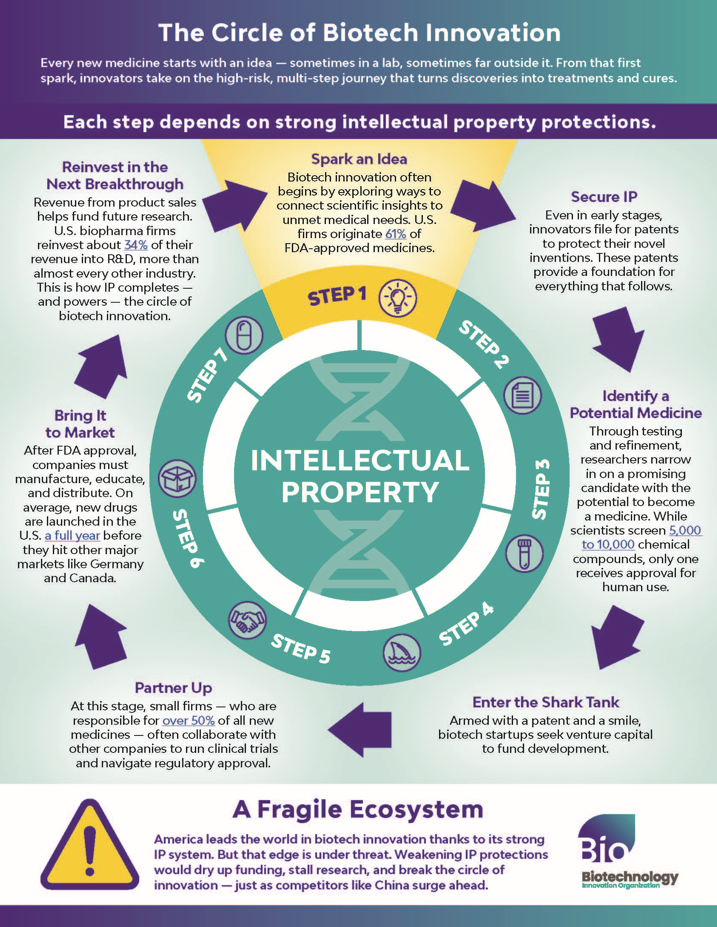
Each Step Depends on Strong IP Protections
Every new medicine starts with an idea - sometimes in a lab, sometimes far outside it. From that first spark, innovators take on the high-risk, multi-step journey that turns discoveries into treatments and cures.
Step 1 - Spark an Idea
Biotech innovation often begins by exploring ways to connect scientific insights to unmet medical needs. U.S. firms originate 61% of FDA-approved medicines.
Step 2 - Secure IP
Even in early stages, innovators file for patents to protect their novel inventions. These patents provide a foundation for everything that follows.
Step 3 - Identify a Potential Medicine
Through testing and refinement, researchers narrow in on a promising candidate with the potential to become a medicine. While scientists screen 5,000 to 10,000 chemical compounds, only one receives approval for human use.
Step 4 - Enter the Shark Tank
Armed with a patent and a smile, biotech startups seek venture capital to fund development.
Step 5 - Partner Up
At this stage, small firms — who are responsible for over 50% of all new medicines — often collaborate with other companies to run clinical trials and navigate regulatory approval.
Step 6 - Bring It to Market
After FDA approval, companies must manufacture, educate, and distribute. On average, new drugs are launched in the U.S. a full year before they hit other major markets like Germany and Canada.
Step 7 - Reinvest in the Next Breakthrough
Revenue from product sales helps fund future research. U.S. biopharma firms reinvest about 34% of their revenue into R&D, more than almost every other industry. This is how IP completes — and powers — the circle of biotech innovation.

BIO IP Conference
November 17-19, 2025・Palm Springs, CA
This is the only conference focused specifically on intellectual property in biotechnology, featuring the latest insights from biotech IP experts, as well as networking events designed to promote discussion and foster relationships among industry colleagues. You'll come away with practical information you can put into use at your organization the next day. The conference is open to both BIO Members and non-members who work or are interested in intellectual property rights.
BIO works with the United States Patent and Trademark Office’s (USPTO) IP attaché program, which strives to improve Intellectual property (IP) systems internationally for the benefit of U.S. stakeholders. IP attachés are posted at U.S. missions around the world to address IP issues arising in their assigned regions.

